Hacked with ultraviolet light

From the smallest to the largest, light plays a fundamental role in providing us with information about our universe. Since the development of spectroscopy (the study of the absorption and emission of light and other radiation by matter) and later the invention of the first operational laser in 1960 by Theodore Maiman, light has been considered the key to our understanding of the universe down to its smallest components.
A research group led by PD Dr Christian Ott in Prof Pfeifer's department at the Max Planck Institute for Nuclear Physics in Heidelberg has now succeeded for the first time in temporally resolving a quantum mechanical dissociation mechanism in oxygen molecules using two different light sources in the extreme ultraviolet spectrum (XUV). The experiment is concerned with how small atoms and molecules interact with high-frequency radiation and how they can be controlled with the electrical fields of the laser pulses. “In our experiment we were able to resolve the dissociation of O2, including quantum tunneling and other processes, from a specific electronically excited state “said Christian Ott.
What role does XUV light play in this? While visible, ultraviolet and infrared light dominate at the Earth's surface, extreme ultraviolet light, i.e. radiation with higher energy, is absorbed by the atmosphere. XUV radiation can be used by laser physics to selectively excite electrons in molecules. This means that certain electrons in the oxygen molecule receive a "shot" of energy, while others remain unaffected.
Atoms are held together by the electrons that are further away from the nucleus. The inner shell electrons are closer to the nucleus and are less susceptible to interactions with other atoms in the molecule. Therefore, inner shell electrons are not normally involved in chemical reactions that take place on the Earth's surface. However, with XUV radiation, scientists can "point" to the inner shell electrons and thus enable new chemical reactions that do not occur in nature.
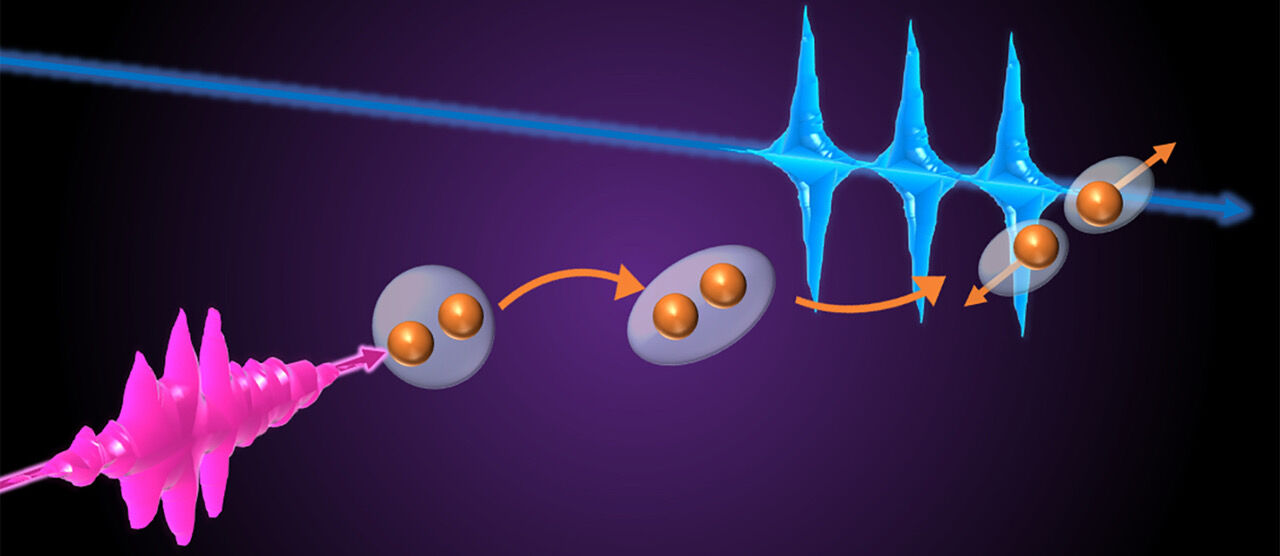
How did the researchers combine two extreme ultraviolet light sources? On the one hand, laser pulses are generated using the High Harmonic Generation (HHG) method, and on the other hand using a free-electron laser (FEL) in Hamburg (FLASH@DESY). In HHG, infrared light is passed through a gas cell and converted into XUV radiation; the HHG pulses have a broad frequency spectrum and a duration of few femtoseconds, one millionth of a billionth of a second. In the FEL, accelerated electrons emit XUV light; the FEL pulses have a much narrower spectrum than the HHG pulses, but a similar duration. In other words: In the visible range, the HHG pulses have a greater variety of different colors, while the FEL pulses have fewer colors.
The electrons of the oxygen molecule are now put into a so-called electronically excited state with the help of FEL pulses. This means that the oxygen molecule becomes unstable and breaks down into its individual atoms, i.e. two O atoms separate from each other. This splitting process is known as dissociation. Until now, it was unclear how quickly this process takes place, as the atoms of the oxygen molecule may also undergo a "quantum tunnelling process". Quantum tunnelling is a quantum mechanical effect in which particles can penetrate energy barriers even though they should not be able to do so according to the laws of classical physics. In addition, the FEL pulses ensure that sufficient O2 molecules are excited.
By adding a second HHG pulse with an adjustable time delay and using the technique of transient absorption spectroscopy (a technique that allows scientists to analyse the changes in light absorption caused by the dissociation of the O2 molecules in this case), the Heidelberg scientists have now experimentally recorded this molecular dissociation, i.e. "photographed" the dissociation process of the oxygen molecule. ”Identifying such processes is relevant for their reactivity to then perform a reaction with another atom or molecule. This also alludes to the possibility to steer chemical reactions by addressing specific electronic transitions” explains Christian Ott
Overall, this enables a better understanding of the interactions of small atmospheric molecules with radiation sources on ultrafast time scales and contributes to a better understanding of molecular fragmentation dynamics by addressing specific electronic transitions in a molecule, Ott further explains.
The results open up a new way to control chemical reactions in molecules with light and to understand and control such reactions. In addition, the same experimental approach can be used to address various questions of quantum dynamics theory in small molecules and molecular dynamics in complex systems.
Original publication:
Magunia, A. et al.
Time-resolving state-specific molecular dissociation with XUV broadband absorption spectroscopy
Science Advances, 22 Nov 2023,
DOI: 10.1126/sciadv.adk1482












