Microlasers as Cell Identifiers

How small can a working laser be? The perhaps surprising answer is ‘very small’ – in fact so small that they can easily be introduced into cells. Prof. Seok-Hyun Yun and his colleagues at Harvard Medical School have succeeded in labelling cells with miniature lasers, which they refer to simply as ‘laser particles’. “We have developed a new generation of microlasers that can be actively taken up by cells,” explains Dr. Nicola Martino, a member of Yun’s research team. These lasers are biocompatible, take up less than 0.1% of the volume of a typical cell, and their presence in cells does not compromise cell function.
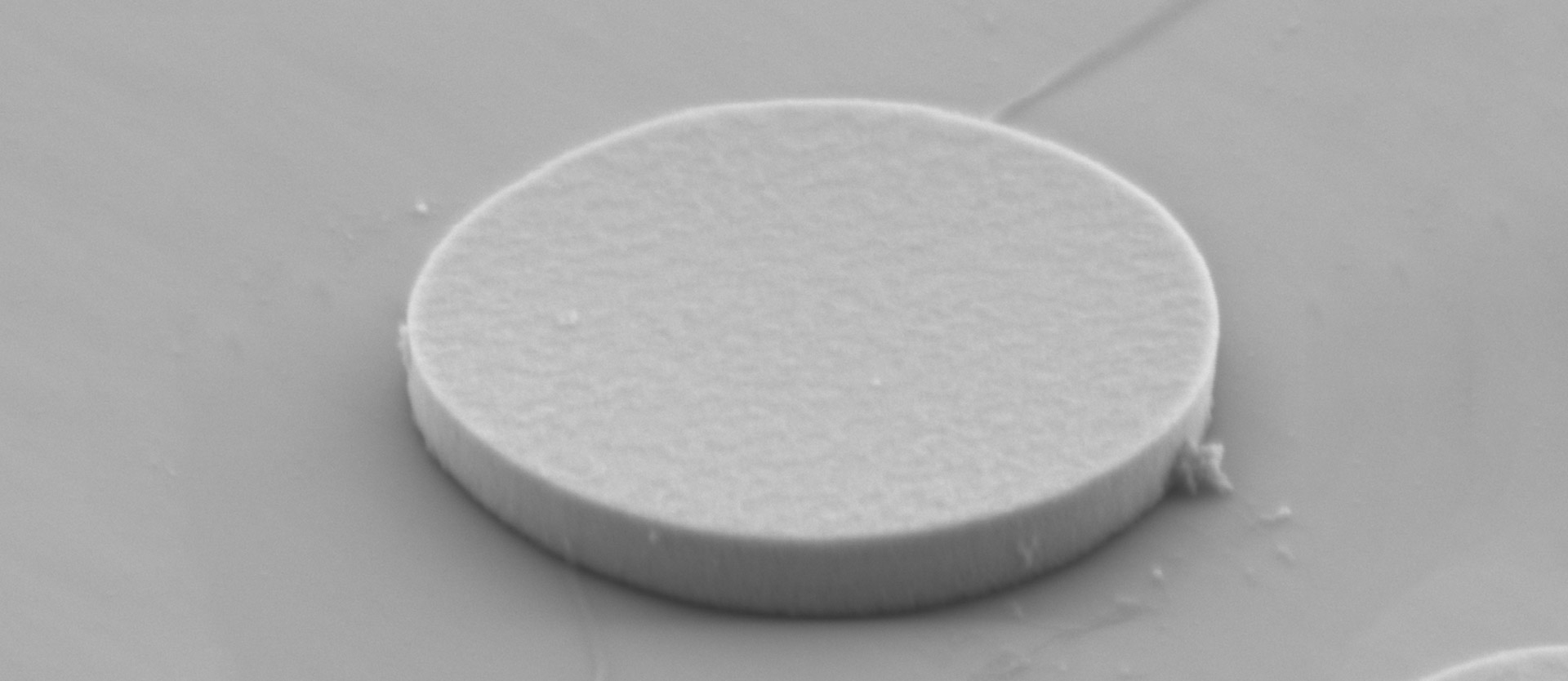
The lasing particles used in this nanosystem are disks made of III-V semiconductors (InAlGaAs or InGaAsP), with a diameter of 2.5 microns and a thickness of 200 nanometers (the diameter of a typical human cell is around 20 microns). In conventional lasers, light emission is excited in a lasing medium by an external energy source and amplified by reflection between two mirrors that form a resonator. The resonators in Yun’s system are of a different type and are known as “whispering-gallery-mode” cavities.
The active laser material is the semiconductor itself. The fluorescent light is confined by total internal reflection within the disc and amplified by stimulated emission. Thus the semiconductor disk functions both as cavity and active material, and each laser particle emits light with a characteristic wavelength that lies between 1200 and 1600 nm. The color of the light emitted is determined by the specific composition of the semiconductor and the precise diameter of the disc, and the researchers can generate more than 400 different wavelengths of light by using batches of discs of different sizes. The laser particles are then coated with a 100 nm thick silica shell, a passive layer that is needed both to protect the disks from degradation due to the aqueous environment and to protect the cells from leaching of toxic ions from the semiconductor core.
In the laboratory, the cells take up the microlasers by the biological process of micropinocytosis, which is a natural process that enables cells to incorporate materials into the cytoplasm by encapsulating them in membrane vesicles. “We use the microlasers to label cells and to trace them in complex biological systems. The lasers provide a barcode, which allows us to distinguish individual cells from one another,” explains Nicola Martino.
The researchers believe that the intracellular lasers can be employed in the fight against cancer. They were able to label thousands of cells in a growing tumor with them, and could follow the movements over the course of several days. “The great advantage of our method is that we can label very many different cells with the lasing particles because the discs emit light with a highly specific wavelength,” Martino explains.
Tumours are extremely heterogeneous in composition and are made up of cells with very different phenotypes. “That is one reason why it is so difficult to eliminate them,” says Martino. “In the future we will be able to observe in detail how cancer cells grow and identify the cells that rapidly give rise to metastases,” he says. “In addition, on the basis of their barcodes we will be able to isolate individual cells more specifically and characterize their genetic profiles”, adds Sheldon J.J. Kwok, Martino’s colleague. Thus, the new cell-labelling technique promises to improve the efficacy and specificity of cancer treatments.
See also: www.nature.com/articles/s41566-019-0489-0












